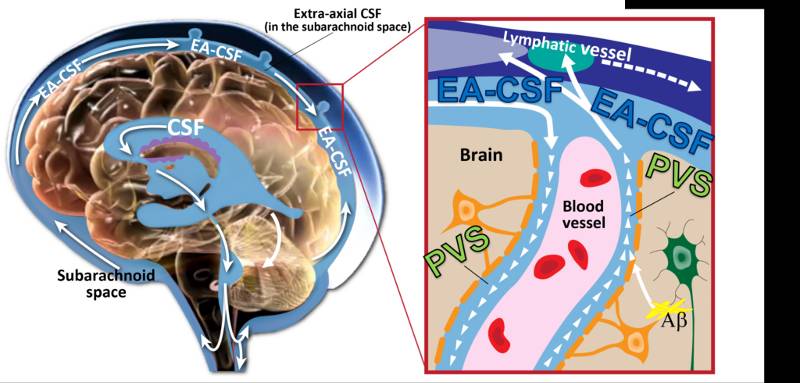
Throughout the day and night, cerebrospinal fluid (CSF) pulses through small fluid-filled channels surrounding blood vessels in the brain, called perivascular spaces, to flush out neuroinflammation and other neurological waste. A disruption to this vital process can lead to neurological dysfunction, cognitive decline, or developmental delays. For the first time, researchers Dea Garic, PhD, and Mark Shen, PhD, both at the UNC School of Medicine’s Department of Psychiatry, discovered that infants with abnormally enlarged perivascular spaces have a 2.2 times greater chance of developing autism compared to infants with the same genetic risk. Their research also indicated that enlarged perivascular spaces in infancy are associated with sleep problems seven to 10 years after diagnosis. “These results suggest that perivascular spaces could serve as an early marker for autism,” said Garic, assistant professor of psychiatry and a member of the Carolina Institute for Developmental Disabilities (CIDD). The researchers studied infants at increased likelihood for developing autism, because they had an older sibling with autism. They followed these infants from 6-24 months of age, before the age of autism diagnosis. Their study, published in JAMA Network Open , found that thirty percent of infants who later developed autism had enlarged perivascular spaces by 12 months. By 24 months of age, nearly half of the infants diagnosed with autism had enlarged perivascular spaces. The Importance of Cerebrospinal Fluid and Sleep The Importance of Cerebrospinal Fluid and Sleep
Starting ten years ago, there has been a resurgence of research on the important functions of CSF in regulating brain health and development. Shen’s lab was the first to report that excessive volume of CSF was evident at 6 months of age in infants who would later develop autism. The current study showed that excessive CSF volume at 6 months was linked to enlarged perivascular spaces at 24 months. Every six hours, the brain expels a wave of CSF that flows through perivascular spaces to remove potentially harmful neuroinflammatory proteins, such as amyloid beta, from building up in the brain. The CSF cleansing process is especially efficient when we are asleep, as the majority of CSF circulation and clearance occurs during sleep. Disrupted sleep, however, can reduce CSF clearance from perivascular spaces, leading to dilation or enlargement, but this has previously only been studied in animal studies or in human studies of adults. This is the first study of its kind in children. Shen, senior author of the JAMA Network Open paper, and Garic hypothesized that CSF abnormalities in infancy would be related to later sleep problems, based on Shen’s earlier research. The current sleep analysis revealed children who had enlarged perivascular spaces at two years of age had higher rates of sleep disturbances at school age. “Since autism is so highly linked with sleep problems, we were in this unique position to examine CSF dynamics and sleep,” said Garic, who is first author of the paper. “It was really striking to observe such a strong association separated by such a long period of time over childhood. But it really shows how perivascular spaces not only have an effect early in life, but they can have long term effects, too.” New Clinical Relevance in Infancy New Clinical Relevance in Infancy
The research was done in conjunction with the Infant Brain Imaging Study (IBIS), a nationwide network of researchers investigating brain development, autism, and related developmental disabilities. The network consists of five universities, of which the University of North Carolina-Chapel Hill is the lead site. For their study, Garic and Shen analyzed 870 MRIs from IBIS to measure excessive CSF volume and enlarged perivascular spaces. MRIs were obtained from babies during natural sleep at six, 12, and 24 months of age to observe changes over time. The infant brain undergoes rapid development over this period. Previously, measurement of perivascular spaces was only thought to be clinically relevant for disorders of aging in older adults, such as in dementia. These findings suggest that younger populations may need to be considered and monitored for these types of brain abnormalities. “Our findings were striking, given that neuroradiologists typically view enlarged perivascular spaces as a sign of neurodegeneration in adults, but this study reported it in toddlers,” said Garic. “This is an important aspect of brain development in the first years of life that should be monitored.” Future Studies and Possibilities Future Studies and Possibilities
Garic and Shen hypothesize that excess CSF volume is stagnant, or clogged, and not circulating through the brain as efficiently as it should. For their next research endeavor, the researchers are planning to once again use MRIs to measure CSF in a sleeping infant’s brain, but this time focusing on the physiology and speed of CSF flow throughout the brain. The research team is also working with other collaborators to quantify the size of perivascular spaces and the severity of behavioral outcomes. The team also plans to extend their research to neurogenetic syndromes associated with autism, such as Fragile X syndrome and Down syndrome. “Collectively our research has shown that CSF abnormalities in the first year of life could have downstream effects on a variety of outcomes, including later autism diagnosis, sleep problems, neuroinflammation, and possibly, other developmental disabilities,” said Shen. This work was supported the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Mental Health (NIMH), National Institute of Environmental Health Sciences (NIEHS), and the Simons Foundation. Other researchers on this project include Joseph Piven, MD; Heather C. Hazlett, PhD; Martin Styner, PhD; Sun Hyung Kim, PhD; Joshua Rutsohn, PhD; and Leigh Anne Weisenfeld, MA; of the University of North Carolina – Chapel Hill; Robert C. McKinstry, MD, PhD; and Kelly N. Botteron, MD; of Washington University in St. Louis; Rebecca Slomowitz, MA; of the University of Denver; Jason Wolff, PhD; of the University of Minnesota; Leigh C. MacIntyre, BSc; of McGill University; Juhi Pandey, PhD; of University of Pennsylvania; and Tanya St. John, PhD; Annette M. Estes, PhD; Robert T. Schultz, PhD; and Stephen R. Dager, MD; of the University of Washington. Media contact: Kendall Daniels , Communications Specialist, UNC Health | UNC School of Medicine
This content was originally published here.