Courtesy of Fred Gage / Salk Institute
Clusters of human cells grafted onto the brains of mice have enabled scientists to probe for the first time how microglia — the brain’s immune cells — develop in an environment that closely models the human brain.
The chimeric mouse model could provide researchers with a more realistic way to study microglia’s roles in brain conditions such as autism, the researchers report in a study published today in Cell.
“It is an important technological step forward to be able to examine the cell signaling and connections of microglia with the new tools of organoid biology,” says Christopher Coe, professor emeritus of biopsychology at the University of Wisconsin-Madison, who was not involved in the work.
Microglia are key players in the brain: They prune synapses, respond to injury and clean up cellular debris. Changes to these cells may even contribute to conditions such as autism and Alzheimer’s disease. In autistic people’s brains, for example, microglia tend to have atypical patterns of gene expression, and the cells are found unusually often in their “active” state.
But scientists have struggled to figure out whether atypical microglia truly drive brain conditions or simply change in response to them, says lead researcher Fred “Rusty” Gage, who leads the Laboratory of Genetics at the Salk Institute in La Jolla, California. Human microglia transplanted into mice do not reflect how the cells develop in the presence of other human cells, Gage says, and their properties are difficult to replicate in a dish.
“If one stops to think about it, it is not normal for cells to grow in a flat monolayer like in the classic lab petri dish,” Coe says.
Other researchers have implanted microglia into organoids, but the clusters of cells do not survive long — possibly because they lack oxygen in the center and become necrotic. In the new work, Gage and his colleagues developed a method for growing human microglia in a human-cell-derived organoid and sustaining them for months by grafting the organoid onto the brain of a mouse — an approach that enables the researchers to test how the cells respond to different manipulations.
Human microglia form in bone marrow and migrate into the brain between 4.5 and 5.5 gestational weeks, so Gage and his colleagues worked to replicate that process. They developed microglia precursors from human pluripotent stem cells and co-cultured them with 35- to 42-day-old human forebrain organoids to enable them to migrate into the cell clusters.
Grafting the organoids onto the brain of a mouse, which provided the cell clusters with essential oxygen and nutrients, enabled the microglia to survive for multiple months — significantly longer than they did when the organoids were in a dish.
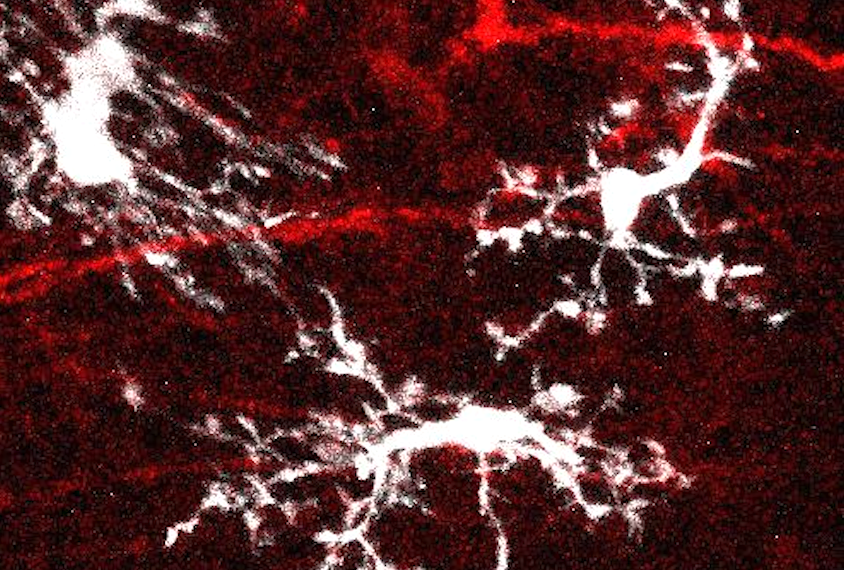
Inside the grafted organoids, the microglia express many of the same genes as they do in people, and that expression changes throughout development in an expected way, RNA sequencing showed. Each of the cells continuously senses its environment and maintains its own territory — its tentacle-like arms pulsing and flitting in every direction — without overlapping with other microglia, lining up with what in vitro studies previously reported.
The microglia also spring into “active mode” to repair damaged tissue and gobble up dead cells, Gage and his colleagues found when they used a laser to kill a neuron in the organoid, showing that the cells are “functional within the tissue” and able to respond to challenges, Gage says.
Microglia inside grafted organoids derived from autistic people who have macrocephaly, an enlarged head, were more likely to take on an “active” morphology than were those that developed inside clusters of cells derived from non-autistic people, the researchers found. And when microglia derived from non-autistic people were instead grown in an organoid derived from an autistic person with macrocephaly, the immune cells took on the “active” characteristics.
That suggests that the observed difference in microglia morphology between autistic and non-autistic people may be a reaction to the brain environment rather than intrinsic to the cells, Gage says.
Those results are an exciting first step toward understanding how human microglia react to their environment, says Auguste Vadisiute, a junior research fellow at St. John’s College, University of Oxford in the United Kingdom, who was not involved in the new study. But because the study looked only at cells derived from autistic people with macrocephaly, she says, the findings likely represent “one small piece” of what is happening in autism. Future studies can use the approach to explore these changes in greater depth, she says.
Coe agrees that more work needs to be done on that front. “With so much variation in presentation and severity across individuals … it is unlikely that there is only one etiological cause” of autism, he adds.
But the new method provides a promising approach for exploring the role of microglia in neurodevelopment, they both say.
One interesting avenue to investigate is how the microglia interact with neurons in their environment and which signaling molecules contribute to different microglia morphology, says Florent Ginhoux, senior principal investigator at the Singapore Immunology Network, who was not involved in the work.
Gage and his colleagues plan to continue studying the cells’ properties, including what happens when they add other glial cells — such as astrocytes — to the organoids, and how changes to microglia contribute to disease.
“What they show is exciting,” Ginhoux says. “But it is really just the beginning.”
This content was originally published here.