Last June, Brady Maher gave a talk about the promise of repurposing existing drugs for people with Pitt-Hopkins syndrome, a rare genetic condition that’s often accompanied by autism. Among the compounds he mentioned was the antihistamine clemastine fumurate, which is available by prescription in the United States, often under the brand name Tavist. In addition to easing allergies, clemastine happens to promote the growth of myelin, the lipid layer that insulates neurons like a plastic sheath around a wire.
About a month later, Maher received an email from a parent who had listened to his talk, delivered at a meeting of the Pitt Hopkins Research Foundation (PHRF) in Chicago, Illinois. Geetika Bajpai’s then-2-year-old son, Aiden, has Pitt-Hopkins syndrome, and Bajpai wanted to give clemastine a try.
Maher, lead investigator at the Lieber Institute for Brain Development in Baltimore, Maryland, was unsure: Clemastine is not approved for young children, and there are no data on how children with Pitt-Hopkins might respond to it. Plus, Maher is a researcher, not a doctor. He suggested she speak to her son’s pediatrician, who agreed to prescribe the drug for Aiden’s allergies — and see if it had any additional effects.
To date, much of what is known about clemastine’s myelin-boosting abilities comes from animal studies. But there are hints that the drug improves brain tissue damage in people with multiple sclerosis, a disease in which the immune system attacks myelin and the cells that produce it, called oligodendrocytes. For example, the drug improves optic-nerve signaling in adults with the condition, according to 2017 clinical trial results.
It’s reasonable to think that increasing myelination might similarly ease traits in some neurodevelopmental conditions, including Pitt-Hopkins syndrome: Mice with mutations seen in people with the condition differentially express oligodendrocyte genes and have fewer of the cells and the myelin they make, Maher found in a 2020 study. Clemastine improves myelination and normalizes brain activity and behavior in the mice, he found in a study published in Brain in April. And the same is true for an experimental compound called sobetirome, which mimics thyroid hormone — necessary for myelination — and for a stripped-down version of the drug, called Sob-AM2, that more readily crosses the blood-brain barrier.
The mounting evidence is enough for researchers to consider a clinical trial of promyelinating drugs in children with Pitt-Hopkins syndrome, Maher says. Both clemastine and Sob-AM2 have advantages and drawbacks: It’s possible clemastine could be trialed more quickly since it has already been approved by the U.S. Food and Drug Administration, Maher says, but it would likely have more side effects than Sob-AM 2; antihistamines can cause drowsiness and slowed cognition. And both drugs likely need toxicology tests in newborn or very young animals.
The animal data look promising but preliminary, says Kimberly Goodspeed, assistant professor of pediatrics, neurology and psychiatry at UT Southwestern Medical Center in Dallas, Texas. Goodspeed was not involved in Maher’s work but treats children with Pitt-Hopkins syndrome.
“It’s a really exciting thing to look at, but it probably needs maybe one more mouse or rat study before you actually take it into humans,” she says. “It’s a really interesting proof of concept.”
Thomas Scanlan read Maher’s 2020 study with great interest. Scanlan, professor of chemical physiology and pharmacology at Oregon Health & Science University in Portland, created sobetirome in 1996 as a treatment for high cholesterol, which is moderated by thyroid hormone. But in the early 2000s, as evidence that thyroid hormone promotes remyelination accumulated, Scanlan pivoted to study the drug in the central nervous system and in 2017 developed Sob-AM2.
In 2018, Scanlan co-founded a company now called Autobahn, which aims to test Sob-AM2 and similar drugs in people with multiple sclerosis or major depression. Both sobetirome and Sob-AM2 help mice that lack myelin to regrow the insulation, he showed in 2019.
In Maher’s Pitt-Hopkins mouse, Scanlan saw yet another opportunity for Sob-AM2.
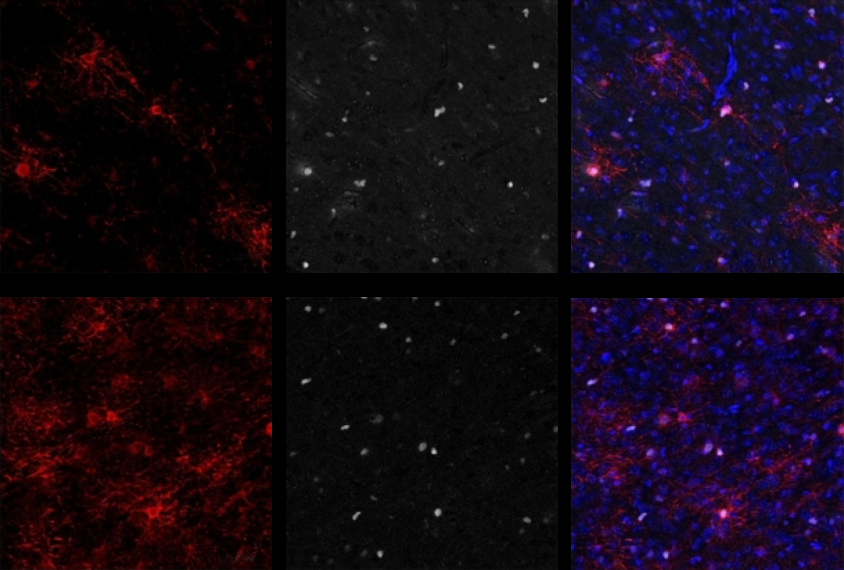
Scanlan emailed Maher a few weeks after the 2020 paper came out, at which point Maher was already studying the use of clemastine and other promyelinating drugs in Pitt-Hopkins mice. Maher agreed to try Scanlan’s drugs in his animals. As the team described in Brain in April, cell cultures from Pitt-Hopkins mice had fewer oligodendrocytes and more oligodendrocyte precursors than did wildtype mice, and bathing the cultures in clemastine reduced this difference. Brain slices from animals that had received clementine injections to the stomach showed the same thing, and the effects lasted nearly seven weeks after the two-week treatment ended.
Axons from the slices of treated Pitt-Hopkins mice showed signs of more ongoing myelination than those of untreated mice — about as much as wildtype mice, the researchers found using transmission electron microscopy. Electrode recordings revealed that treated mice have a more typical ratio of signals traveling along myelinated versus unmyelinated axons than untreated ones. (Maher didn’t test this ratio in the controls.)
Untreated model mice also behaved unusually in an open area: They scurried around much more than wildtypes and frequently moved to the center of the space, indicating an atypical lack of anxiety. Both behaviors normalized with clemastine treatment. Administering Sob-AM2 instead had the same effects, and sobetirome restored wildtype levels of oligodendrocyte and oligodendrocyte precursors in the animals.
Anyone looking to trial Sob-AM2 in people would need to license it from Oregon Health & Science University, which holds the patent. (The patent on Sobetirome expired in 2016, but the drug wouldn’t be a good clinical candidate for brain conditions given its difficulty crossing the blood-brain barrier, Scanlan says.) He would also need to submit an Investigational New Drug application to the Food and Drug Administration.
The drug has never been tested for safety in people, and there are no data on what doses should be used. “It’s something to think about in the future,” Scanlan says. “But it’s not going to happen tomorrow.” (Autobahn has no current plans to study Sob-AM2 in Pitt-Hopkins syndrome, Scanlan says.)
Clemastine, on the other hand, could go into an academic trial now, he says. Such a trial could provide further “proof of concept” for using myelinating drugs for Pitt-Hopkins, Scanlan says, though clemastine’s sedating side effects and limited brain uptake make it less than ideal for regular use. “Neither one of them is perfectly keyed up to go into clinical development.”
Axon aid: After clemastine injection, axons from Pitt-Hopkins mice have more myelin, marked with yellow and white arrows, than mice that don’t receive the drug.
Even so, families with children who have the condition may be eager to join a trial. About 1,100 people worldwide have Pitt-Hopkins syndrome, according to a census managed by PHRF. And the parents are often amenable to trying new treatments, Goodspeed says. After Maher tested an experimental compound in Pitt-Hopkins mice in 2016, for example, PHRF touted the study as a “potential treatment.” And several families wanted to try the blood pressure drug nicardipine following a 2020 study showing the medicine improved social and repetitive behaviors in model mice, Goodspeed says.
Children who have already tried clemastine would likely be excluded from any trials, Maher says. But there are 406 children known to have Pitt-Hopkins in the U.S., and, as far as Maher knows, only 3 have taken clemastine. The numbers might be higher in Europe, where the drug is available over the counter.
Balancing the needs of families and the demands of clinical rigor is difficult, Goodspeed says. If children are taking a drug off-label, it can disrupt formal trials — particularly because parents are often wary of disclosing such use to researchers, she says.
“You don’t want stop anything, because if something seems safe and effective, that should be their right to try it,” she says. “But we have to be nimble and thoughtful as we’re moving forward.”
Bajpai, at least, felt prepared to safely to test the waters: As a pharmacist, she knows how to measure doses and watch for side effects. For six months, Aiden took 1.34 milligrams of clemastine daily, compared with the 5.36 milligrams adults took twice daily in the multiple sclerosis trial. (He now takes it only as needed for allergies.)
Anecdotally at least, Aiden seems more alert and aware of his surroundings on the drug, Bajpai says. The boy’s physical therapist and teacher, neither of whom knew about the treatment, mentioned the changes too. Two other families trying the drug saw similar results, Bajpai says.
“That’s huge, right?” she says. “From someone who won’t respond his name to someone who you feel like you’re actually playing with now.”
For all the promise, though, there’s no guarantee the drugs will be a success. At least one family told Maher about a negative experience with clemastine: The child became hyperactive after taking the drug and had to stop using it. “These kids have obviously much different brain chemistry than we do,” Maher says, noting that antihistamines typically cause drowsiness.
Maher also warns parents that progress is difficult to monitor without objective outcome measures. And because there hasn’t been a natural-history study of Pitt-Hopkins syndrome, little is known about typical development in children with the condition.
Maher says he plans to do more behavioral tests in the Pitt-Hopkins mice, such as a water-maze task that measures learning and memory. If clemastine improves learning and memory in mice, clinical trials in people could measure similar outcomes. He also wants to test whether the drug eases the breathing problems that occur in both model mice and people with Pitt-Hopkins syndrome.
Stem cells from people with Pitt-Hopkins syndrome struggle to differentiate into oligodendrocytes, Maher says. But it is not known whether children with Pitt-Hopkins have reduced white matter like the model mice.
Maher says he also plans to test clemastine in mouse models of other genetic syndromes linked to autism, which could expand the number of people available to test the drug in a clinical trial. Some animal studies suggest other autism-linked mutations cause myelin deficits: Mice with mutations in CHD8, for example, have thin myelin sheaths that seem to impede electrical signals, an independent team showed in 2020.
“We’re not saying myelination is the only problem,” Maher says. “This is just one piece of the puzzle and maybe something that would be easy to help.”
This content was originally published here.