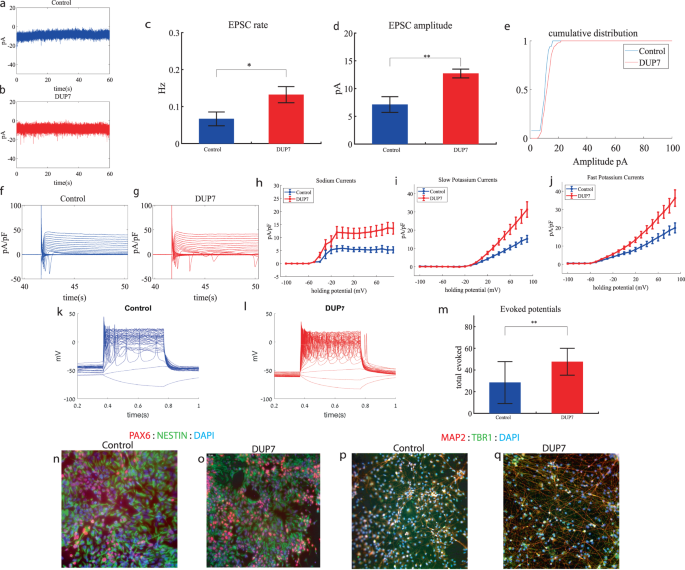
Dup7 cortical neurons display increased sodium and potassium currents, increased synaptic activity and hyperexcitability early in the differentiation
We performed whole-cell patch clamp experiments 5 weeks (day 34) after the start of the differentiation of 16 Dup7-mutant neurons and 13 control neurons derived from a first-degree relative of the same gender. In a voltage clamp mode, EPSC recordings were performed by holding the cell at −60 mV. We observed an increase in the rate of EPSCs of the mutant neurons compared to the controls (0.13 ± 0.08 Hz in Dup7-mutant neurons and 0.07 ± 0.07 Hz in the control neurons, p = 0.03), as shown in Fig. 1a–c. Figure 1a, b presents representative traces, while Fig. 1c represents the average over all the recordings. In addition, a significant increase in the mean amplitude of the EPSCs was observed. The Dup7-mutant neurons had a larger amplitude compared to the control neurons (12.72 ± 2.86 pA for the mutant neurons and 7.12 + 5.09 pA for the control neurons (p = 0.002, (Fig. 1d)). The cumulative distribution of the EPSC amplitudes for Dup7-mutant neurons is slightly right-shifted compared to control neurons indicating larger amplitudes of EPSCs (Fig. 1e).
a A representative trace of (EPSCs measured in control cortical neurons at 5 weeks post-differentiation. b A representative trace of EPSCs measured in a dup7-mutant neuron at 5 weeks post-differentiation. c The mean rate of synaptic events was higher in the dup7-mutant neurons compared to control neurons (p = 0.029). d The average amplitude of EPSCs was increased in the dup7-mutant neurons (p = 0.002). e The cumulative distribution of the amplitude of EPSCs is slightly right-shifted in the dup7-mutant neurons, indicating an increase in the amplitudes. f A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in control neurons. g A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in dup7-mutant neurons. h The average sodium currents in dup7-mutant neurons is increased compared to control neurons (p = 0.004). i The average slow potassium currents in dup7-mutant neurons is increased compared to control neurons (p = 0.03). j The average fast potassium currents is increased in dup7-mutant compared to control neurons (p = 0.01). k A representative recording of evoked action potentials in a current-clamp mode of a control neuron. l A representative recording of evoked action potentials in a current-clamp mode of a dup7-mutant neuron. m The total number of evoked action potentials is larger in dup7-mutant neurons compared to control neurons (p = 0.006). n, o A representative image of control n and mutant o NPCs that were immunostained for DAPI, PAX6, and Nestin. p, q A representative image of control p and mutant q neurons that were immunostained for DAPI, MAP2, and TBR1.
a A representative trace of (EPSCs measured in control cortical neurons at 5 weeks post-differentiation. b A representative trace of EPSCs measured in a dup7-mutant neuron at 5 weeks post-differentiation. c The mean rate of synaptic events was higher in the dup7-mutant neurons compared to control neurons (p = 0.029). d The average amplitude of EPSCs was increased in the dup7-mutant neurons (p = 0.002). e The cumulative distribution of the amplitude of EPSCs is slightly right-shifted in the dup7-mutant neurons, indicating an increase in the amplitudes. f A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in control neurons. g A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in dup7-mutant neurons. h The average sodium currents in dup7-mutant neurons is increased compared to control neurons (p = 0.004). i The average slow potassium currents in dup7-mutant neurons is increased compared to control neurons (p = 0.03). j The average fast potassium currents is increased in dup7-mutant compared to control neurons (p = 0.01). k A representative recording of evoked action potentials in a current-clamp mode of a control neuron. l A representative recording of evoked action potentials in a current-clamp mode of a dup7-mutant neuron. m The total number of evoked action potentials is larger in dup7-mutant neurons compared to control neurons (p = 0.006). n, o A representative image of control n and mutant o NPCs that were immunostained for DAPI, PAX6, and Nestin. p, q A representative image of control p and mutant q neurons that were immunostained for DAPI, MAP2, and TBR1.
Next, we recorded in voltage clamp mode the sodium and potassium currents. We observed a significantly larger normalized sodium current in the Dup7-mutant neurons compared to the control neurons (F (1,38) = 9.43, p = 0.004). Representative traces of the recordings are shown in Fig. 1f (control) and 1g (mutant). The average sodium currents are presented in Fig. 1h. In addition, we observed increased slow and fast potassium currents (normalized by the capacitance) in the Dup7-mutant neurons compared to controls (F (1,14) = 5.61; p = 0.03 for the slow potassium currents and F (1,14) = 8.36; p = 0.01 for the fast potassium currents) over the 10–80 mV range (Fig. 1h–j). We next measured the number of evoked action potentials in a current clamp mode as a measure of the neuronal excitability. We observed a hyperexcitability pattern for the Dup7-mutant neurons compared to control neurons. The total number of evoked potentials (see “Materials and methods”) for the Dup7-mutant neurons was 47.63 ± 49.61; for the control neurons, it was 28.5 ± 69.48 (p = 0.006). A representative example is presented in Fig. 1k (control) and 1l (Dup7), and the average over all recordings is shown in Fig. 1m. Spike shape analysis (see “Materials and methods”) is presented in Table S2. Examples of ICC images for control and mutant lines are shown in Fig. 1n, o (typical NPC markers PAX6 and NESTIN) and 1p, q (neuronal markers MAP2 and the cortical marker TBR1).
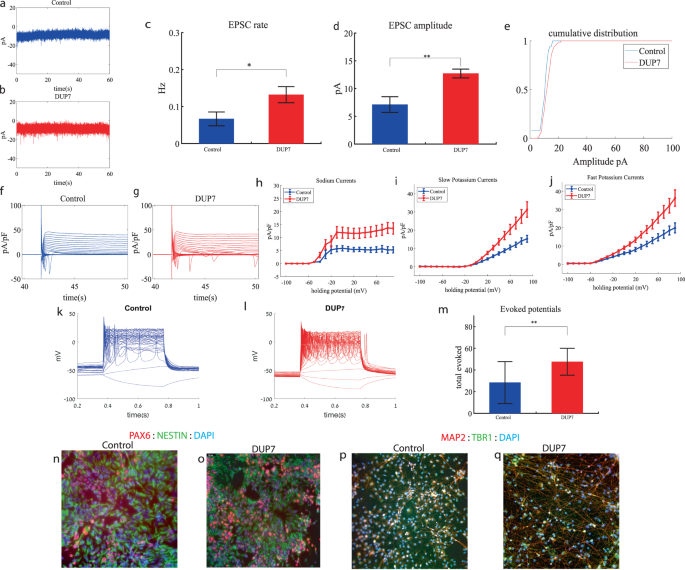
GRIN2B cortical neurons display increased sodium and potassium currents and hyperexcitability early in the differentiation
We performed whole-cell patch clamp experiments 3 weeks (day 19) after the start of the differentiation of 12 GRIN2B-mutant neurons and 5 weeks (day 27) of 7 control neurons. In a voltage clamp mode, EPSC recordings were performed by holding the cell at −60 mV. We observed an increase in the rate of EPSCs of the mutant neurons compared to the controls (0.39 ± 0.27 Hz in GRIN2B-mutant neurons and 0.17 ± 0.2 Hz in the control neurons, p = 0.03) as shown in Fig. 2a–c. Figure 2a, b presents representative traces, while Fig. 2c represents the average over all the recordings. No significant difference in the mean amplitude of the EPSCs was observed (Fig. 2d). The cumulative distribution of the EPSC amplitudes was similar between the control and GRIN2B-mutant neurons (Fig. 2e).
a A representative trace of EPSCs measured in control cortical neurons at 5 weeks post-differentiation. b A representative trace of EPSCs measured in a GRIN2B-mutant neuron at 3 weeks post-differentiation. c The mean rate of synaptic events was higher in the GRIN2B-mutant neurons (p = 0.03). d The mean amplitude of EPSCs was increased but not significantly different in the GRIN2B-mutant neurons. e The cumulative distribution of the amplitude of the EPSCs of GRIN2B-mutant and control neurons looks similar. f A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in control neurons. g A Representative trace of sodium and potassium currents recorded in voltage-clamp in GRIN2B-mutant neurons. h The average sodium currents in GRIN2B-mutant neurons is severely increased compared to control neurons (p = 0.006). I The average slow potassium currents in GRIN2B-mutant neurons is increased compared to control neurons (p = 0.04). j The average fast potassium currents in GRIN2B-mutant neurons is increased compared to control neurons (p = 0.03). k A representative recording of evoked action potentials in a current-clamp mode of a control neuron. l A representative recording of evoked action potentials in a current-clamp mode of a GRIN2B-mutant neuron. m The total number of evoked action potentials is larger in the GRIN2B-mutant neurons compared to control neurons (p = 0.003). n, o A representative image of control (n) and mutant (o) NPCs that were immunostained for DAPI, PAX6, and Nestin. p, q A representative image of control (p) and mutant (q) neurons that were immunostained for DAPI, MAP2, and TBR1.
a A representative trace of EPSCs measured in control cortical neurons at 5 weeks post-differentiation. b A representative trace of EPSCs measured in a GRIN2B-mutant neuron at 3 weeks post-differentiation. c The mean rate of synaptic events was higher in the GRIN2B-mutant neurons (p = 0.03). d The mean amplitude of EPSCs was increased but not significantly different in the GRIN2B-mutant neurons. e The cumulative distribution of the amplitude of the EPSCs of GRIN2B-mutant and control neurons looks similar. f A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in control neurons. g A Representative trace of sodium and potassium currents recorded in voltage-clamp in GRIN2B-mutant neurons. h The average sodium currents in GRIN2B-mutant neurons is severely increased compared to control neurons (p = 0.006). I The average slow potassium currents in GRIN2B-mutant neurons is increased compared to control neurons (p = 0.04). j The average fast potassium currents in GRIN2B-mutant neurons is increased compared to control neurons (p = 0.03). k A representative recording of evoked action potentials in a current-clamp mode of a control neuron. l A representative recording of evoked action potentials in a current-clamp mode of a GRIN2B-mutant neuron. m The total number of evoked action potentials is larger in the GRIN2B-mutant neurons compared to control neurons (p = 0.003). n, o A representative image of control (n) and mutant (o) NPCs that were immunostained for DAPI, PAX6, and Nestin. p, q A representative image of control (p) and mutant (q) neurons that were immunostained for DAPI, MAP2, and TBR1.
Next, we recorded in voltage clamp mode the sodium and potassium currents. We observed a significantly larger normalized sodium current in the GRIN2B-mutant neurons compared to the control neurons (F (1,38) = 14.1, p = 0.0006). Representative traces of the recordings are shown in Fig. 2f (control) and 2g (mutant). The average sodium currents are presented in Fig. 2h. In addition, we observed larger slow and fast potassium currents (normalized by the capacitance) in the GRIN2B-mutant neurons compared to controls (F (1,16) = 4.6, p = 0.04 for the slow potassium currents and F (1,16) = 5.39, p = 0.03 for the fast potassium currents) over the 0–80 mV range (Fig. 2h–j).
We next measured the number of evoked action potentials in current clamp mode as a measure of the neuronal excitability. We observed a hyperexcitability pattern for the GRIN2B-mutant neurons compared to control neurons. The total number of evoked potentials (see “Materials and methods”) in the GRIN2B-mutant neurons was 38.86 ± 10.17, and in the control neurons, it was 22.37 ± 19.5 (p = 0.003). A representative example is presented in Fig. 2k (control) and 2l (GRIN2B), and the average over all recordings is presented in Fig. 2m. Furthermore, we observed a significant increase in GRIN2B-mutant neurons’ spike amplitude compared to control neurons (41.2 ± 12.5 mV in GRIN2B-mutant neurons and 20.5 ± 14.01 mV in control neurons, p = 0.008); further spike shape analysis is presented in Table S2. Examples of ICC images for control and mutant lines are shown in Fig. 2n, o (typical NPC markers PAX6 and NESTIN) and 2p, q (neuronal markers MAP2, the cortical marker TBR1).
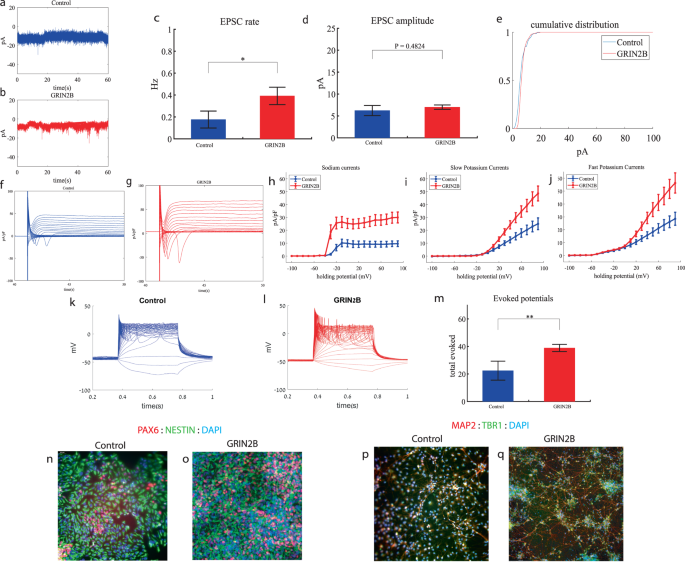
SHANK3 cortical neurons display increased sodium and slow potassium currents, a drastic increase in synaptic activity and hyperexcitability early in the differentiation
We performed whole-cell patch clamp experiments 5 weeks (days 29–32) after the start of the differentiation of 21 SHANK3-mutant and 17 control neurons. In a voltage clamp mode, EPSC recordings were performed by holding the cell at −60 mV. We observed a significant increase in the rate of EPSCs of the mutant neurons compared to the controls (0.28 ± 0.36 Hz in SHANK3-mutant neurons and 0.08 ± 0.06 Hz in the control neurons, p 0.003) as shown in Fig. 3a–c. Figure 3a, b presents representative traces, while Fig. 3c represents the average over all the recordings. In addition, a drastic increase in the mean amplitude of the EPSCs was observed. The SHANK3-mutant neurons had a larger amplitude compared to the control neurons (10.145 ± 3.26 pA for the mutant neurons and 5.29 + 1.65 pA for the control neurons, p = 1.15e−5 (Fig. 3d)). The cumulative distribution of the EPSC amplitudes for SHANK3-mutant neurons is right shifted compared to control neurons indicating larger amplitudes of EPSCs (Fig. 3e).
a A representative trace of excitatory postsynaptic currents (EPSCs) that were measured in control cortical neurons at 5 weeks post-differentiation. b A representative trace of EPSCs measured in a SHANK3-mutant neuron at 5 weeks post-differentiation. c The mean rate of synaptic events was higher in the SHANK3-mutant neurons (p = 0.003). d The average amplitude of EPSCs was increased in the SHANK3-mutant neurons (p = 1.15e−5). e The cumulative distribution of the amplitude of EPSCs of SHANK3-mutant is right-shifted, indicating an increase in the amplitudes. f A Representative trace of sodium and potassium currents recorded in voltage-clamp in control neurons. g A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in SHANK3-mutant neurons. h The average sodium currents in SHANK3-mutant neurons is increased compared to control neurons (p = 0.04). I The average slow potassium currents in SHANK3-mutant neurons is increased compared to control neurons (p = 0.03). j The average fast potassium currents look similar and not significantly different in SHANK3-mutant compared to control neurons. k A representative recording of evoked action potentials in a current-clamp mode of control neurons. l A representative recording of evoked action potentials in a current-clamp mode of SHANK3-mutant neurons. m The total number of evoked action potentials is larger in SHANK3-mutant neurons compared to control neurons (p = 0.03). n, o A representative image of control (n) and mutant (o) NPCs that were immunostained for DAPI, PAX6, and Nestin. p, q A representative image of control (p) and mutant (q) neurons that were immunostained for DAPI, MAP2, and TBR1.
a A representative trace of excitatory postsynaptic currents (EPSCs) that were measured in control cortical neurons at 5 weeks post-differentiation. b A representative trace of EPSCs measured in a SHANK3-mutant neuron at 5 weeks post-differentiation. c The mean rate of synaptic events was higher in the SHANK3-mutant neurons (p = 0.003). d The average amplitude of EPSCs was increased in the SHANK3-mutant neurons (p = 1.15e−5). e The cumulative distribution of the amplitude of EPSCs of SHANK3-mutant is right-shifted, indicating an increase in the amplitudes. f A Representative trace of sodium and potassium currents recorded in voltage-clamp in control neurons. g A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in SHANK3-mutant neurons. h The average sodium currents in SHANK3-mutant neurons is increased compared to control neurons (p = 0.04). I The average slow potassium currents in SHANK3-mutant neurons is increased compared to control neurons (p = 0.03). j The average fast potassium currents look similar and not significantly different in SHANK3-mutant compared to control neurons. k A representative recording of evoked action potentials in a current-clamp mode of control neurons. l A representative recording of evoked action potentials in a current-clamp mode of SHANK3-mutant neurons. m The total number of evoked action potentials is larger in SHANK3-mutant neurons compared to control neurons (p = 0.03). n, o A representative image of control (n) and mutant (o) NPCs that were immunostained for DAPI, PAX6, and Nestin. p, q A representative image of control (p) and mutant (q) neurons that were immunostained for DAPI, MAP2, and TBR1.
Next, we recorded in voltage clamp mode the sodium and potassium currents. We observed a significantly larger normalized sodium current in the SHANK3-mutant neurons compared to the control neurons F (1,36) = 4.51, p = 0.04. Representative traces of the recordings are shown in Fig. 3f (control) and 3g (mutant). The average sodium currents are presented in Fig. 3h. In addition, we observed increased slow, but not fast, potassium currents (normalized by the capacitance) in the SHANK3-mutant neurons compared to controls, (F (1,10) = 5.68; p = 0.03) over the 40–90 mV range (Fig. 3h–j).
We next measured the number of evoked action potentials in current clamp mode as a measure of the neuronal excitability. We observed a hyperexcitability pattern for the SHANK3-mutant neurons compared to control neurons. The total number of evoked potentials (see “Materials and methods”) for the SHANK3-mutant neurons was 31.32 ± 39.8; for the control neurons, it was 18.4 ± 22.96 (p = 0.03). A representative example is presented in Fig. 3k (control) and 3l (SHANK3) and the average over all recordings are presented in Fig. 3m. Furthermore, we observed a more depolarized threshold (higher) in the SHANK3-mutant neurons compared to control neurons (30.4 ± 5.6 mV in the SHANK3-mutant neurons and 25.5 ± 4.2 mV in control neurons, p = 0.02); further spike shape analysis is presented in Table S2. Examples of ICC images of control and mutant lines are shown in Fig. 3n, o (typical NPC markers PAX6 and NESTIN) and 3p, q (neuronal markers MAP2, the cortical marker TBR1).
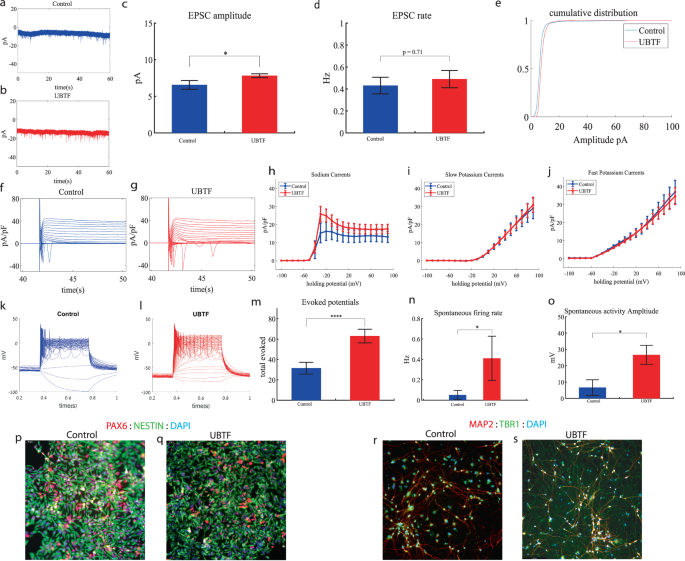
UBTF cortical neurons display increased sodium currents, an increase in synaptic amplitude, an increase in spontaneous activity and hyperexcitability early in the differentiation
We performed whole-cell patch clamp experiments 5 to 6 weeks (days 32–37) after the start of the differentiation of 22 UBTF-mutant neurons and 5 to 6 weeks (days 32–37) after the beginning of the differentiation of 18 control neurons. In a voltage clamp mode, EPSC recordings were performed by holding the cell at −60 mV. Figure 4a, b presents representative traces, while Fig. 4c represents the average EPSC amplitude over all the recordings. The EPSC rate of the UBTF-mutant neurons is slightly similar to the control neurons (Fig. 4c). We observed an increase in the mean amplitude of the EPSCs. The UBTF-mutant neurons had a larger amplitude compared to control neurons it was 7.81 ± 1.16 pA for the mutant neurons and 6.55 + 2.39 pA for control neurons, p = 0.02 (Fig. 4d). The cumulative distribution of the EPSC amplitudes for UBTF-mutant neurons is slightly right-shifted compared to control neurons indicating larger amplitudes of EPSCs (Fig. 4e).
a A representative trace of excitatory postsynaptic currents (EPSCs) that were measured in control cortical neurons at 6 weeks post-differentiation. b A representative trace of EPSCs measured in a UBTF-mutant neuron at five weeks post-differentiation. c The mean rate of synaptic events was not significantly different in UBTF-mutant neurons compared to control neurons. d The average amplitude of EPSCs was increased in the UBTF-mutant neurons (p = 0.02). e The cumulative distribution of the amplitude of EPSCs of UBTF-mutant neurons is slightly right-shifted, indicating an increase in the amplitudes. f A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in control neurons. g A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in UBTF-mutant neurons. h The average sodium currents in UBTF-mutant neurons is increased compared to control neurons (p < 0.05). I The average slow potassium currents look similar in the UBTF-mutant and control neurons. j The average fast potassium currents look identical in the UBTF-mutant and control neurons. k A representative recording of evoked action potentials in the current-clamp mode of control neurons. l A representative recording of evoked action potentials in a current-clamp mode of UBTF-mutant neurons. m The total number of evoked action potentials is higher in the UBTF-mutant neurons compared to control neurons (p = 5.56e−4). n The mean rate of spontaneous action potentials was significantly higher in UBTF-mutant neurons compared to control neurons (p = 0.013). o The mean amplitude of spontaneous action potentials in UBTF-mutant neurons was larger compared to control neurons (p = 0.012). p, q A representative image of control (p) and mutant (q) NPCs that were immunostained for DAPI, PAX6, and Nestin. r, s A representative image of control (r) and mutant (s) neurons that were immunostained for DAPI, MAP2, and TBR1.
a A representative trace of excitatory postsynaptic currents (EPSCs) that were measured in control cortical neurons at 6 weeks post-differentiation. b A representative trace of EPSCs measured in a UBTF-mutant neuron at five weeks post-differentiation. c The mean rate of synaptic events was not significantly different in UBTF-mutant neurons compared to control neurons. d The average amplitude of EPSCs was increased in the UBTF-mutant neurons (p = 0.02). e The cumulative distribution of the amplitude of EPSCs of UBTF-mutant neurons is slightly right-shifted, indicating an increase in the amplitudes. f A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in control neurons. g A Representative trace of sodium and potassium currents recorded in a voltage-clamp mode in UBTF-mutant neurons. h The average sodium currents in UBTF-mutant neurons is increased compared to control neurons (p < 0.05). I The average slow potassium currents look similar in the UBTF-mutant and control neurons. j The average fast potassium currents look identical in the UBTF-mutant and control neurons. k A representative recording of evoked action potentials in the current-clamp mode of control neurons. l A representative recording of evoked action potentials in a current-clamp mode of UBTF-mutant neurons. m The total number of evoked action potentials is higher in the UBTF-mutant neurons compared to control neurons (p = 5.56e−4). n The mean rate of spontaneous action potentials was significantly higher in UBTF-mutant neurons compared to control neurons (p = 0.013). o The mean amplitude of spontaneous action potentials in UBTF-mutant neurons was larger compared to control neurons (p = 0.012). p, q A representative image of control (p) and mutant (q) NPCs that were immunostained for DAPI, PAX6, and Nestin. r, s A representative image of control (r) and mutant (s) neurons that were immunostained for DAPI, MAP2, and TBR1.
Next, we recorded in voltage clamp mode the sodium and potassium currents. We observed a significantly larger normalized sodium current in the UBTF-mutant neurons compared to the control neurons F (1,28) = 5.58, p = 0.03 over the −50–90-mV range. Representative traces of the recordings are shown in Fig. 4f (control) and 4 g (mutant). The average sodium currents is presented in Fig. 4h. An ANOVA test indicated no significant differences in the slow and fast potassium currents (Fig. 4h–j).
We next measured the number of evoked action potentials (see “Materials and methods”). For the UBTF-mutant neurons, it was 62.95 ± 31.56 and for the control neurons, it was 31.46 ± 22.3 (p = 5.56e−4). A representative example is presented in Fig. 4k (control) and 4 l. (UBTF) and the average over all recordings are presented in Fig. 4m. Furthermore, we observed a significant increase in UBTF-mutant neurons’ spike amplitude compared to control neurons (50 ± 17.05 mV in the UBTF-mutant neurons and 36.04 ± 18.01 mV in control neurons, p = 0.03). Besides, we observed a narrower spike in the UBTF-mutant neurons compared to the control neurons (3.3 ± 1.5 ms in the UBTF-mutant neurons and 12.5 ± 17.8 ms in the control neurons, p = 0.003); further spike shape analysis is presented in Table S2. The spontaneous neuronal activity (spontaneous action potentials) was measured in a holding potential of −45 mV. We observed a significant increase in the spontaneous activity rate (p = 0.013) and amplitudes (p = 0.012) in the UBTF-mutant neurons compared to control neurons (Fig. 4n, o). Examples of ICC images of control and mutant lines are shown in Fig. 4p, q (typical NPC markers PAX6 and NESTIN) and 4r, s (neuronal markers MAP2, the cortical marker TBR1).
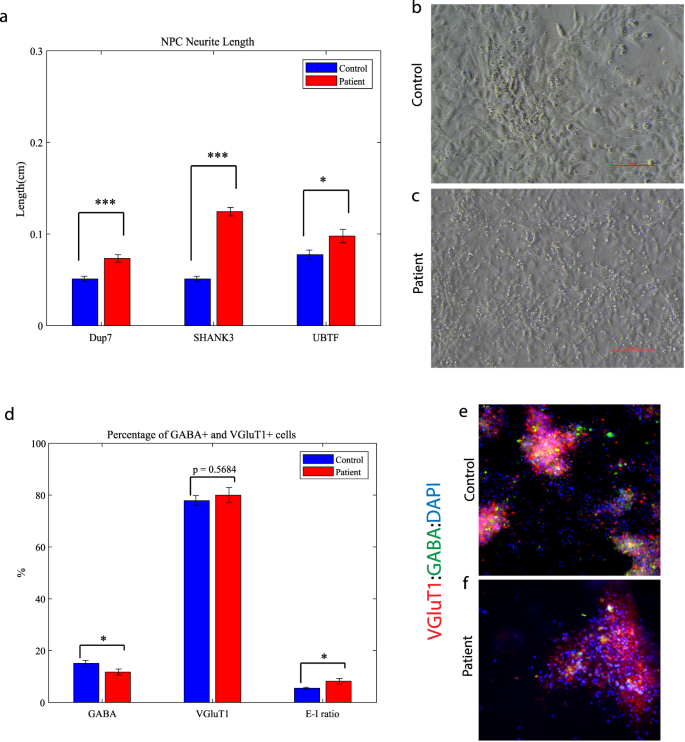
Mutant neural progenitor cells exhibit longer neurite lengths compared to controls, alongside with decreased GABA-positive neurons in ASD-related mutant neuronal cultures
We observed that the lengths of neurites of neural progenitor cells (NPCs) in mutant groups were significantly increased compared to control groups (Fig. 5a). This difference was determined by analyzing brightfield images captured at ×20 magnification and measuring the length of neurites using a standardized methodology (see “Materials and methods”). Specifically, the average length of NPC neurites in the Dup7-mutant NPCs (left) was 0.12 ± 0.02 cm compared to control NPCs was 0.07 ± 0.02 cm (p = 4.36e−04), in the SHANK3-mutant NPCs (middle) the average length was 0.09 ± 0.03 cm compared to control NPCs 0.07 ± 0.02 cm (p = 6.75e−08) and in the UBTF-mutant NPCs it was 0.09 ± 0.03 cm compared to control NPCs 0.05 ± 0.01 cm (p = 0.01), as determined by Wilcoxon signed-rank test. Example images of control (b) and patient (c) are shown in Fig. 5b, c.
a A longer neurites in Dup7-NPCs (left) p = 4.36e−04, SHANK3-NPCs (middle) p = 6.75e−08 and UBTF-NPCs (right) p = 0.01 mutants compared to control groups. b The averages of the percentage of GABA-expressing neurons (left) out of the total neurons in the ASD-mutant neuronal cultures are significantly lower than the percentage of GABA-expressing neurons in the control neuronal cultures (p = 0.04), The averages of the percentage of VGluT1-expressing neurons (middle) out of the total neurons in the ASD-mutant neuronal cultures are similar to the percentage of VGluT1-expressing neurons in the control neuronal cultures (p = 0.56) and a higher E-I ratio (right) in ASD-mutant neuronal cultures compared to control cultures (p = 0.03). c, d A representative image of mutant (c) and control (d) neurons that were immunostained for DAPI, GABA, and VGluT1.
a A longer neurites in Dup7-NPCs (left) p = 4.36e−04, SHANK3-NPCs (middle) p = 6.75e−08 and UBTF-NPCs (right) p = 0.01 mutants compared to control groups. b The averages of the percentage of GABA-expressing neurons (left) out of the total neurons in the ASD-mutant neuronal cultures are significantly lower than the percentage of GABA-expressing neurons in the control neuronal cultures (p = 0.04), The averages of the percentage of VGluT1-expressing neurons (middle) out of the total neurons in the ASD-mutant neuronal cultures are similar to the percentage of VGluT1-expressing neurons in the control neuronal cultures (p = 0.56) and a higher E-I ratio (right) in ASD-mutant neuronal cultures compared to control cultures (p = 0.03). c, d A representative image of mutant (c) and control (d) neurons that were immunostained for DAPI, GABA, and VGluT1.
This content was originally published here.