This study was approved by the Ethical Committee for Biomedical Research at the University of Leuven, KU Leuven (S61358) in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent from the parents and assent from the child were obtained prior to the study.
Participants
Eighty children with a formal diagnosis of ASD, aged between 8–12 years, were recruited through the Leuven Autism Expertise Centre at the Leuven University Hospital between July 2019 and January 2021. Also, 40 age- and sex-matched peers without ASD (control) were recruited. The adopted sample size (80 ASD, 40 controls) will allow detecting medium-to-large diagnostic-related effects (independent samples) with alpha set at 0.05 and power at 0.8 (estimated using G*Power).
The main inclusion criteria comprised a clinical diagnosis of ASD (only for children with ASD), premenstrual girls, intelligence quotient (IQ) above 70, and native Dutch speaker. The main exclusion criteria comprised a history of any neurological disorder (stroke, concussion, epilepsy, etc.), any significant physical disorder (liver, renal, cardiac pathology), or any neuropsychiatric diagnosis (only for control children). The diagnosis of ASD was established by a multidisciplinary team (child psychiatrist and/or expert neuropediatrician, psychologist, speech/language pathologist and/or physiotherapist) based on the strict criteria of the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders) [41].
In addition, the Autism Diagnostic Observation Schedule (ADOS-2) [42] was acquired (Table 1). For all children, estimates of intelligence were acquired using four subtests of the Wechsler Intelligence Scale for Children, Fifth Edition, Dutch version [43] (Table 1).
Children were also thoroughly characterized on autism symptom domains, using the parent-reported versions of the Social Responsiveness Scale, second edition (SRS-2) [44, 45] and the Repetitive Behavior Scale-Revised (RBS-R) [46]. Also, the parent-rated Child Behavior Checklist (CBCL) was obtained to assess behavioral problems and competences [47] (see Supplementary Methods for detailed descriptions of the adopted questionnaires).
As outlined in Table 1, groups were matched on age and sex, although verbal and performance IQ were overall higher in the control group. As expected, children of the ASD group demonstrated significantly higher scores on the parent-rated SRS-2 and RBS-R, indicating more social impairments and more frequent expressions of restricted and repetitive behavior (p < .001, Table 1). The parent-rated CBCL also showed diagnosis-related effects, indicating more severe deficits in the ASD group, compared to the control group (p < .05, Table 1).
Note that the (biological) data collected for the current report were part of a larger clinical study including additional neurophysiological assessments (see also next section).
Oxytocin and cortisol salivary concentrations
For each child, salivary samples were acquired at two time points: (i) an AM sample, acquired at home, in the morning, within 30 min after awakening and before breakfast; and (ii) a PM sample, acquired in the afternoon, after completion of an experimental session performed at the Leuven University hospital. Importantly, the PM sample was collected within 30 min after finalizing an experimental test session, consisting of a semi-structured social interaction with an unknown experimenter, which could be experienced as moderately stressful, especially to the pediatric participants (see Supplementary Methods).
Salivary samples were collected using Salivette cotton swabs (Sarstedt AG & Co.). For the analysis of salivary oxytocin levels, the commercial enzyme immunoassay oxytocin ELISA kit of Enzo Life Sciences, Inc. was used, similar to prior studies [48,49,50]. All sample extraction and concentration procedures were conducted in accordance with the manufacturer’s instructions. Measurements were performed on undiluted samples (100 μl), and sample concentrations were calculated according to plate-specific standard curves.
Analysis of the cortisol levels was performed using the commercial enzyme immunoassay Cortisol ELISA kit of Salimetrics, Europe. Measurements were performed on undiluted samples (25 μl), and sample concentrations were calculated according to plate-specific standard curves. More detailed information regarding the salivary collection procedures and analyses is provided in Supplementary Methods.
DNA methylation of the oxytocin receptor gene
Additional salivary samples were obtained via the Oragene DNA sample collection kit (DNA Genotek Inc., Canada) to address epigenetic variations in the level of methylation (DNAm) at two CpG sites (-934 and -924) of OXTR (hg19, chr3:8,810,729–8,810,845).
After data collection, 200 ng DNA was extracted from the samples, and bisulfite was converted following the manufacturer’s protocol (EZ-96 DNAm Kit, Zymo Research, Irvine, CA, USA). Bisulfite-converted DNA was stored at −80 °C until further analysis. Next, the levels of methylation at two CpG sites (i.e., -934 and -924) of OXTR (hg19, chr3:8,810,729–8,810,845) were determined using Pyrosequencer (Qiagen, Hilden, Germany) and analyzed using Pyromark Q96 software. Laboratory procedures and analyses were conducted in accordance with the manufacturer’s protocols and software settings (e.g. for determining unreliable samples) [47]. Protocols for the PCR amplification and Pyrosequencing analysis were adapted from Krol et al. (2019) [51]. More information regarding the adopted PCR primers can be found in Supplementary Methods.
Data handling and statistical procedures
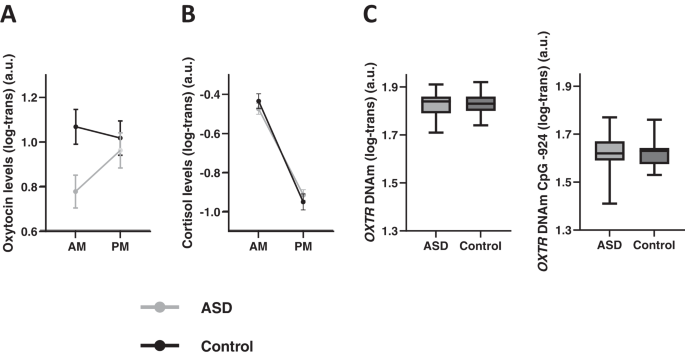
Prior to analysis, hormonal and DNAm data were log-transformed (log10) to deal with skewed data. Next, diagnosis-related differences were assessed by subjecting oxytocin and cortisol hormonal data to repeated-measure analyses-of-variance (ANOVA) with the between-subject factor group (ASD, control) and the within-subject factor time point (AM, PM) (see Supplementary Tables 1–2 for full ANOVA models).
OXTR DNAm data were subjected to independent sample t-tests to examine diagnosis-related differences in DNAm at CpG site -924 and -934.
Pearson correlation analyses were performed to assess associations between the two hormonal systems in children with or without ASD and between hormonal levels and OXTR DNAm. Exploratory, also correlation analyses between oxytocin hormonal levels and behavioral scales (SRS-2, RBS-R, CBCL) were examined.
Finally, to examine the potential impact of person-dependent factors, such as age, verbal and performance IQ, secondary analyses were performed, including these variables as dimensional covariates in the performed statistical analyses. Overall, for all reported analyses, the observed results patterns remained qualitatively similar with inclusion of these covariates (data not reported).
All statistical analyses were executed with SPSS (version 28.0, IBM). Due to the exploratory nature of the reported analyses, all results are reported at an uncorrected statistical threshold of p > .05.
This content was originally published here.