Essentially opposite paths in fetal brain development may explain two major subtypes of autism. In one of these subtypes, an unusually high number of excitatory neurons in a key brain region leads to large heads, or macrocephaly, which affects roughly 20 percent of people with autism; in the other, a decreased number of the same cells in that area leads to more typical head sizes, a new study finds.
This fundamental biological difference suggests that “therapeutic avenues may be drastically different for these subtypes,” says lead investigator Flora Vaccarino, professor of neuroscience at Yale University. “That in turn could explain why drug treatments for autism so far are failing.”
The opposite brain development paths found in this research may both lead to autism because they are each a case of imbalance, says investigator Alexej Abyzov, associate professor of biomedical informatics at the Mayo Clinic in Rochester, Minnesota.
“Imagine walking on two legs — if one is much shorter, you will fall, and it doesn’t matter which one is shorter,” he says. “The same holds true here — you can have different characteristics, but the key feature is imbalance.”
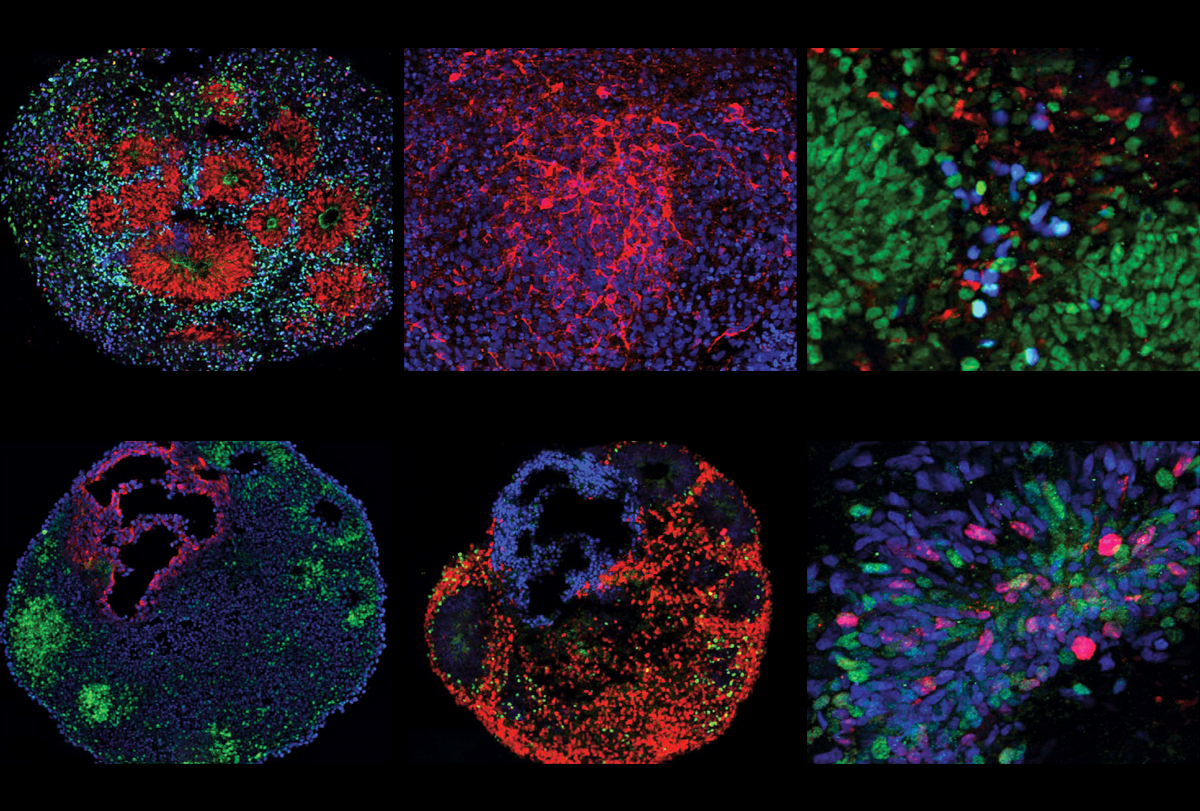
Vaccarino and her colleagues generated brain organoids by using skin and bladder cells from autistic boys and their non-autistic fathers. Eight of the autistic boys had macrocephaly and five had typical-sized heads.
The researchers also compared the whole genomes of the boys and their fathers. They did not find any rare mutations in the boys that might help explain their autism, which suggests the alterations in the autistic boys’ brain development stem from common variants, epigenomic modifications or both.
Such “idiopathic” cases are diverse in nature, and it has remained unclear what biological underpinnings they might have in common. Yet the organoids derived from autistic boys with macrocephaly all showed a relative excess of excitatory neurons early in the development of the cortical plate, the scientists found. This structure gives rise to the cerebral cortex.
These organoids also showed an increase in the expression of some genes associated with excitatory neurons, Vaccarino says. By contrast, organoids grown from autistic boys with typical head sizes showed a decrease in the expression of some of the same genes in the same type of neurons.
The work helps address “an overarching question of how many ways you can get to similar endpoints in autism,” says Tom Nowakowski, associate professor of anatomy, psychiatry and neurological surgery at the University of California at San Francisco, who did not take part in this research. The new study suggests there could be overlap in the genetic dysregulation of neural progenitor cells across different instances of autism, he adds.
Vaccarino, Abyzov and their colleagues had previously found that organoids derived from autistic boys with macrocephaly displayed changes in cells that control neural activity. The new findings may further support the hypothesis that an imbalance between excitatory and inhibitory signaling may underlie autism, they say.
Future research should examine more people, Vaccarino says. She and her colleagues also plan to look for links between mutations in the genomes of these people and relevant molecular pathways “that may be responsible for the imbalances we see,” she says. “Eventually we will know the cause of every single patient’s autism, we think.”
For the time being, Vaccarino adds, this research is limited by the fact that “We can’t grow these organoids for very long.” She says she hopes that in the future, techniques will advance such that it will be possible to look at later timepoints.
Nowakowski concurs, noting “These are very early developmental changes, some of which may or may not persist into postnatal stages of development.” To investigate the long-term outcomes of these fetal effects, one avenue might be to examine postmortem tissues of autistic people regardless of whether they have macrocephaly, he says. “That would be tremendously informative, I think.”
The scientists detailed their findings on 10 August in Nature Neuroscience.
This content was originally published here.