New ‘prime editing’ CRISPR-based tools supply gains in delivery and safety, and can even flag their own potential mistakes, according to a spate of recent studies.
As such, they might be tapped to modify autism-linked mutations and ease some of the condition’s traits, says Yang Yang, associate professor of medicinal chemistry and molecular pharmacology at Purdue University in West Lafayette, Indiana. Autism often results from multiple mutations, but “recent large-scale human genetic studies have identified a growing number of autism cases caused by monogenic mutations in a single gene.”
Prime editing, developed in 2019, offers advantages over conventional CRISPR systems. For instance, instead of making edits by cutting two strands of DNA at once and using a cell’s own error-prone repair machinery to fix these breaks, prime editing depends on a modified reverse transcriptase enzyme that creates more reliably-mended single-strand breaks, one at a time.
But despite the benefit, prime editors — at roughly 6,300 DNA letters long — have been too big for delivery via adeno-associated viruses (AAVs), limiting their utility.
To solve that problem, the method’s inventors — scientists led by David Liu of Harvard University — devised a way to split the tool and pack it into two AAVs. Once inside a cell, the halves come together again.
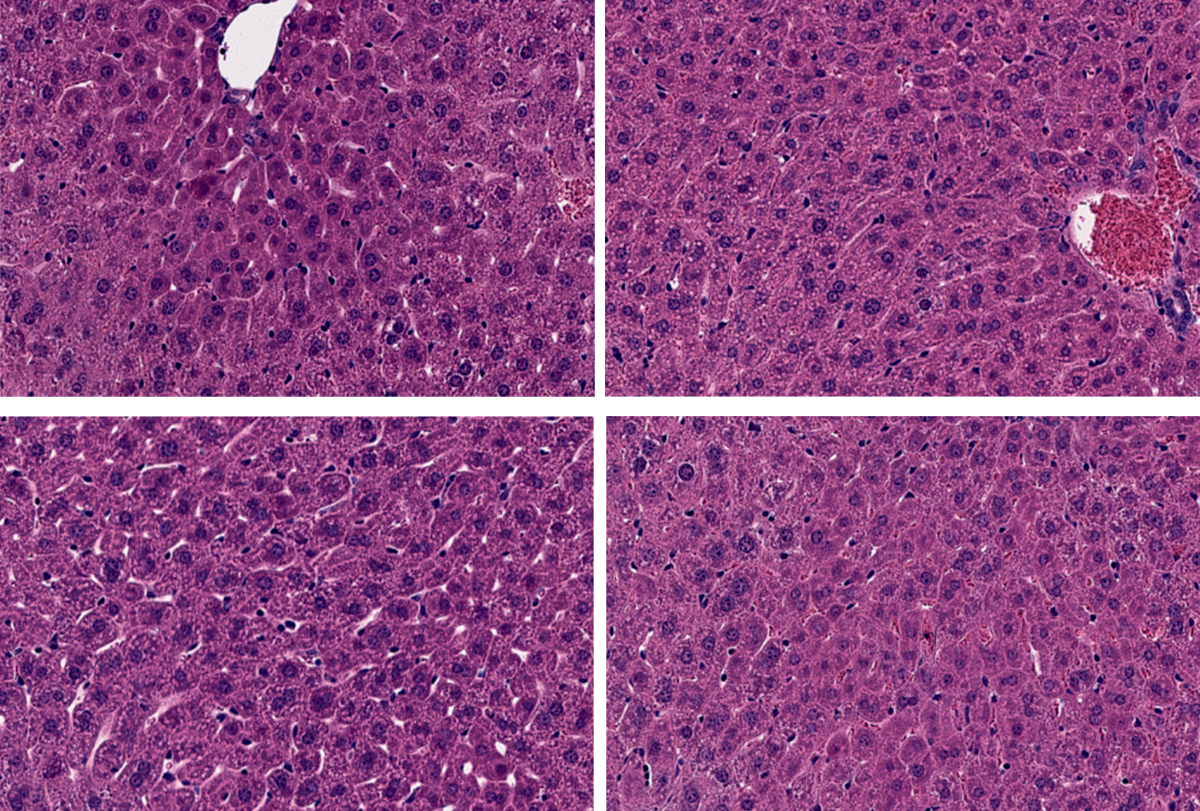
The team used the dual-virus strategy in mice to install mutations in star-shaped astrocytes that prior work suggests could protect against Alzheimer’s disease, and in liver cells to lower cholesterol levels and defend against coronary artery disease. The viruses showed no signs of being toxic or delivering unintended edits, according to a study published 4 May in Nature Biotechnology.
The tactic edited up to 42 percent of cells in the animals’ cerebral cortex, 46 percent of cells in the liver, and 11 percent of cells in the heart, outperforming previous split prime editors. It is also the first prime-editing tool deployed in postnatal brain and heart tissue, the team notes.
Gene editing in the brain has proven difficult “largely due to its low efficiency,” says Jerzy Szablowski, assistant professor of bioengineering at Rice University in Houston, who did not take part in the study.
But this new strategy is efficient enough in the brain to “result in clinically significant outcomes,” says Yang, who also did not participate in the work. “I anticipate that this inspiring paper will open up a large array of studies utilizing prime editor to correct disease-causing mutations in preclinical models, including those found in children with autism.” Future research should move tests of this approach to non-human primates, he adds.
For research applications, mice engineered to encode and express a prime editor in every cell appear to offer another solution to the delivery problem. In this set up, the prime editor remains silent until activated by an enzyme called CRE recombinase, delivered in a lipid nanoparticle via injection.
Before CRISPR and other gene-editing tools, developing a line of lab mice to investigate a single mutation could take months or years, says Tyler Jacks, the David H. Koch professor of biology at the Massachusetts Institute of Technology, who works with Liu and pioneered this approach. “With this new model, we can make virtually any mutation of interest in any gene or regulatory sequence of interest in any cell of interest. I like to say now that we are limited only by our creativity.”
Jacks’ lab focuses on cancer but “the prime editor mouse that we describe can be used to introduce mutations in genes relevant to any process, including genes involved in autism,” he notes.
In experiments, he and his team modeled lung and pancreatic cancer, injecting CRE-recombinase into select tissues along with several different guide RNAs to mutate K-RAS or p53 — linked to about 30 and 60 percent of all human cancers, respectively. They detailed their findings online 11 May in Nature Biotechnology.
A major concern with any gene editor is unwanted changes — particularly in the case of a gene therapy that seeks to edit a large number of cells.
Yet another new prime editing method called PE-tag makes it easier to spot off-target effects across the genome by adding a tag wherever it makes a change. Its creators — led by an independent team of scientists at the University of Massachusetts Chan Medical School and their colleagues — showed they could identify a tag’s location not just in DNA extracted from cells, but also in lab-grown human embryonic kidney cells and in the livers of live mice.
“While the prediction of off-target sites can be achieved via computational approaches, prediction always needs careful testing,” says Yang, who did not take part in this study. “The exciting part of this paper is the new strategy to experimentally identify [a] potential off-target site in the genome.”
Prime editing systems designed as therapies for Tay-Sachs disease, cystic fibrosis and Rett syndrome had 43, 20 and 24 potential off-target sites, respectively, the team found. But they could lower that number by shortening the lifespan of the prime editors. They detailed their findings online 8 May in Nature Methods.
“For any prime editing to move into clinical translation, understanding the off-target profile would be critically important to evaluate the potential side effects of that particular editing in humans,” Yang says. “The technology developed in this paper would help to facilitate this critical step of evaluating side effects.”
This new strategy was mostly tested on human embryonic kidney cells, Yang notes. Future research should examine if prime editing has similar or different off-target profiles in mature cells, including neurons whose genetic activity may differ significantly.
This content was originally published here.