Deleting sticky molecules called neuroligins from astrocytes in mice has no effect on the cells’ shape or on synapse formation, a new preprint claims. The findings are at odds with those of a high-profile paper published in 2017 in Nature.
But the 2017 team, along with researchers not involved in either study, say that — among other shortcomings — the new work is inconclusive, because it does not sufficiently demonstrate that the neuroligins were deleted.
“As a general case, it’s exceedingly difficult to convincingly demonstrate that something is not doing something, because it really critically hinges on being 100 percent sure you have completely abolished that gene product on the protein level,” says Peter Scheiffele, professor of cellular and developmental neurobiology at the Biozentrum of the University of Basel in Switzerland, who was not involved in either study. “The burden of proof to be really convinced that you have successfully ablated your target gene is very high.”
Neuroligins (NLGNs) are abundantly expressed on the surface of neurons, where they hold synapses together, and they have been implicated in autism.
The 2017 work showed that astrocytes express NLGN1, NLGN2 and NLGN3. Partially suppressing them via an RNA technique stunts the astrocytes’ growth in culture and alters their hallmark star-like shape, according to the study. Mice missing NLGN2 from some of their astrocytes formed fewer excitatory synapses around those astrocytes in the visual cortex. When NLGN2 was deleted from about half of the astrocytes in the mice, neuronal excitation dropped by about 25 percent.
The 2017 results “seemed to validate the idea that astrocytes are an essential component of synapse formation by identifying a molecule that seemed to be essential for that,” says Thomas Südhof, professor of molecular and cellular physiology and neurosurgery at Stanford University in California, who led the new preprint but wasn’t involved in the 2017 work.
That finding raised suspicions for Südhof, he says, because NLGN2 had previously been found only in inhibitory synapses. So he set out to test the finding by completely deleting NLGN1, NLGN2 and NLGN3 from the astrocytes of mice.
The preprint’s null result is unlikely to be explained by incomplete deletion of the neuroligins, as Scheiffele and others suggest, Südhof says. “Genetic deletions are the gold standard in biology,” he wrote in an email to Spectrum, responding to the critiques. “This is what I would call a ‘last gasp’ concern — a remote possibility to explain a result people don’t like.”
The preprint’s results do not rule out the possibility that astrocyte neuroligins might have some influence on synaptic function. “Our only claim in our paper is that we test whether neuroligins in astrocytes are essential for excitatory or inhibitory synapses,” Südhof says. “Our conclusion is that they are not.”
He and his colleagues posted their preprint on bioRxiv in April and published it as a reviewed preprint in eLife on 23 June. According to one of these anonymous public reviews, the preprint is “a very clear and strong paper that is supported by rigorous experiments.”
But another anonymous review noted that the study did not provide direct evidence that all three neuroligins were efficiently deleted from astrocytes, leaving the study “somewhat vulnerable to the criticism, however unlikely, that their lack of synaptic effects may be a consequence of incomplete neuroligin deletion, rather than a true lack of effect of astrocytic neuroligins.” As of this story’s publication date, none of the reviewers had responded to Spectrum’s request for comment.
The authors of the 2017 study declined to discuss the matter by phone, preferring to respond to questions over email. Lead investigator Cagla Eroglu, professor of cell biology and neurobiology at Duke University in Durham, North Carolina, wrote in an email to Spectrum that the new preprint is not as conclusive as it claims to be.
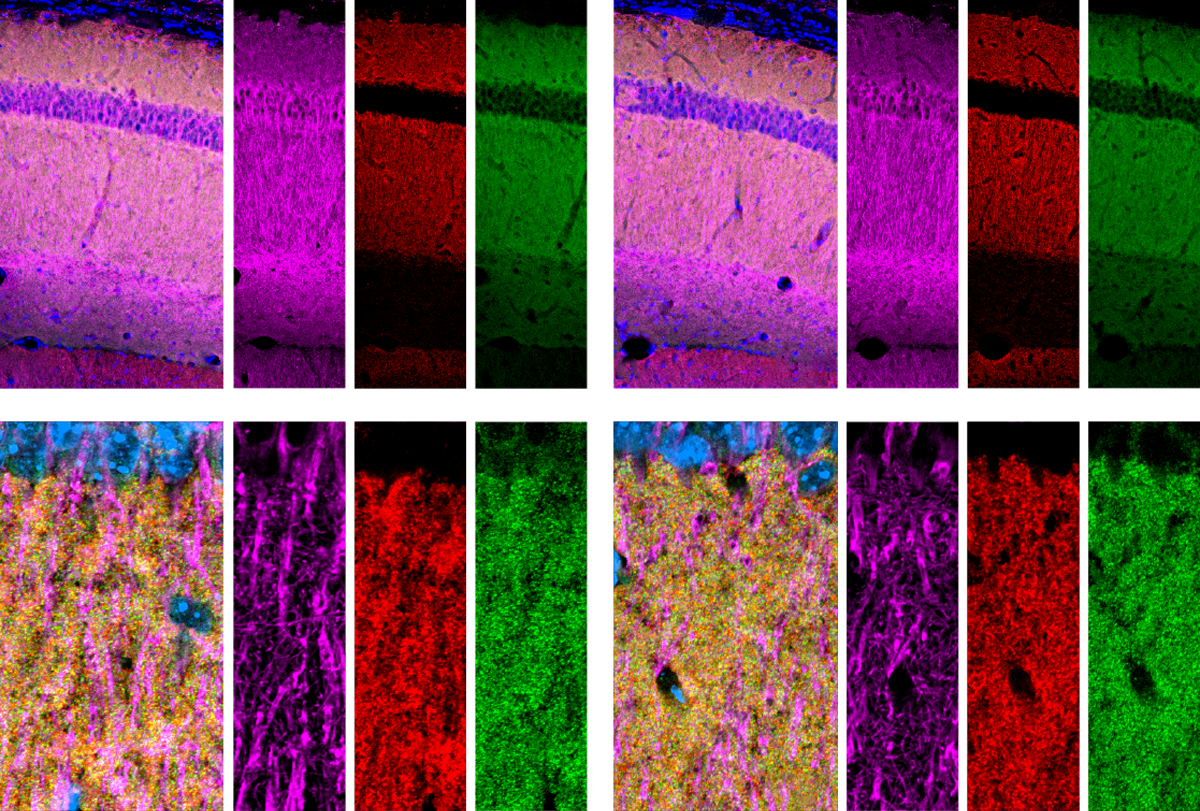
Little difference: Astrocytes in the hippocampus (top row) or visual cortex (bottom row) of mice show no change in shape or volume following the deletion of neuroligins (right two columns) compared with controls (left two columns).
The reason has to do with the genetic techniques involved, Eroglu says. According to the preprint, Südhof’s team bred their mice so that the neuroligins of interest could be deleted by an enzyme called Cre-recombinase when they treated the animals with a drug called tamoxifen. And in a separate experiment, Südhof’s team crossed the same Cre mice with mice that are engineered to express a red protein, tTomato, where Cre is active.
The red protein appeared in more than 80 percent of astrocytes in the hippocampus and about 90 percent in the visual cortex in this second experiment, the preprint shows, which indicates that the neuroligins in question would be ablated with the same efficiency in the knockout experiment, Südhof says.
Eroglu’s team used a similar Cre-based technique to delete NLGN2 in mice engineered to express the same red reporter protein. But her team took an additional step to verify the suppression of NLGN2, she wrote via email: NLGN2 RNA levels dropped significantly, but not completely, in about 50 percent of astrocytes.
Südhof’s team should confirm their triple deletion in some way, too, others say. “It’s very well known that different loci recombine at very different rates, and getting a marker turned on does not mean you will actually get a knockout of the gene of interest,” Scheiffele says. On top of that, “the more alleles you combine to achieve ablation, the less efficiently each individual one will be ablated.”
That’s because mice have two copies of NLGN1 and NLGN2 (female mice have two copies of NLGN3, as well, whereas male mice have only one copy). In the best-case scenario, assuming all alleles recombine at the same 80 percent efficiency as the gene that expresses the tTomato reporter, the chances of deleting all copies of the three genes is about 1 in 3 for male mice and 1 in 4 for female mice, Scheiffele estimates.
The levels of the tTomato reporter indicate only how likely Cre is to act on DNA in a given cell type. It does not guarantee that a targeted gene, or three of them, will be deleted in any given cell, says Bill Buaas, associate director of Operational Efficiency and Platform Development at The Jackson Laboratory in Bar Harbor, Maine, which houses, distributes and engineers mutant mouse strains.
And because NLGN genes have two alleles, “really for them to get loss of function in these genes, they have to get both copies in the same cell,” Buaas adds. “That’s a taller order.”
For these reasons, Südhof’s team would need to verify the deletion of the neuroligins using antibodies that would glom onto the proteins and stain them if they are present, Buaas says. “What you really want to do is say: Are the [astrocytes] missing my protein of interest?” Buaas explains. “That’s an important set of experiments to do.”
Scheiffele and others agree, saying the verification would be difficult but not impossible to do.
“Everything goes down to ‘please show me how much protein is at the cell surface of an astrocyte, and is this protein removed,’” says Frank Kirchhoff, professor of molecular physiology at the University of Saarland in Germany.
Südhof disagrees, saying it is not technically possible to measure neuroligins in astrocytes in the way that Buaas and Kirchhoff suggest. He insists that his team’s technique is solid. “We have shown before that these three neuroligin genes are efficiently recombined by Cre recombinase and that there is no incompleteness in the deletion,” he wrote in an email to Spectrum, referring to a 2015 paper and 2017 paper in which he and colleagues deleted the three neuroligins from neurons.
But different cell-specific Cre mouse strains “can behave completely differently from an efficiency perspective,” Buaas says. “You cannot assume that they are going to delete at the same frequencies.” And, he says, in Südhof’s 2017 paper the team infected mice directly with Cre rather than crossing them with a Cre mouse strain. “This is like comparing apples to oranges,” Buaas wrote in an email to Spectrum.
The preprint’s null result makes it even more important to verify that deletion has occurred, Buaas says. “I think if you’re being really disciplined about the scientific process, concluding something from no effect is extremely dangerous.”
Both Eroglu’s 2017 study and Südhof’s preprint share a weakness, Scheiffele and Kirchhoff say: Neither investigated the neuroligins’ physical presence in astrocytes. “Without really showing directly the changes in the protein level — the protein change at the single-cell level — for me, it is hard to really take all the data as they are,” Kirchhoff says. “I think both have to do electron microscopy with immunoglobulin staining to show that the protein’s expressed at the surface” of astrocytes.
Eroglu says her team’s 2017 paper shows that NLGN2 protein is expressed in astrocytes from mice, based on an experiment that used confocal microscopy with immunoglobulin staining. “Neuroligin antibodies for staining are poor. Therefore, we are developing other methodologies to detect these proteins,” she wrote in an email.
She remains stalwart — astrocyte neuroligins deserve the field’s attention. “We are investigating the functions of astrocytic neuroligins in depth,” she wrote. “Some of these studies are close to completion, and we look forward to sharing our exciting new findings soon.”
Cite this article: https://doi.org/10.53053/SYOS2516
This content was originally published here.