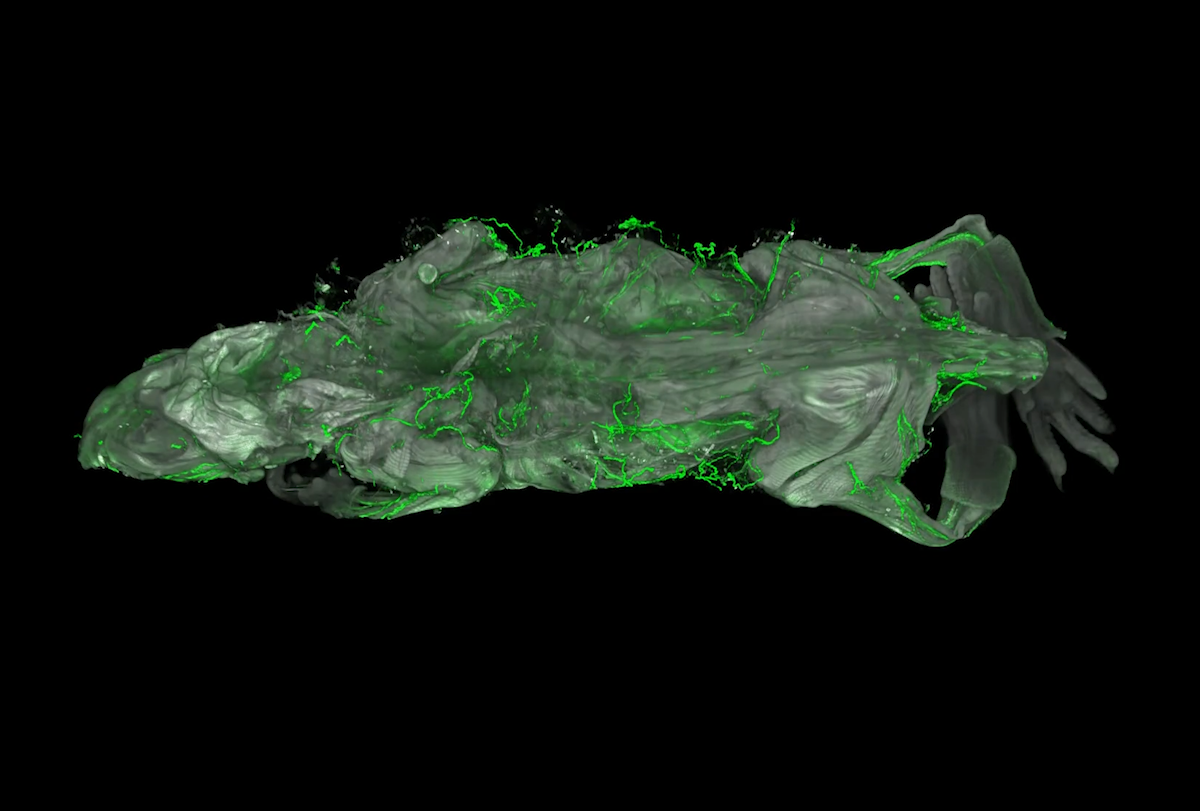
By turning mice transparent and using fluorescent antibodies to label specific cell types, researchers have created exquisitely detailed whole-body maps of intact neurons, immune cells, blood vessels and lymph networks.
The technology can help scientists understand how the nervous system interacts with other parts of the body, says lead investigator Ali Ertürk, director of Helmholtz Munich’s Institute for Tissue Engineering and Regenerative Medicine in Germany. “In the context of autism, it could provide a whole-body perspective of the neurological alterations associated with the condition.”
The new technique, known as wildDISCO and described last week in Nature Biotechnology, is the latest advance in tissue clearing, in which scientists bathe organs and even entire animals in chemicals postmortem to render them transparent — opening a view into specific cells or proteins tagged with fluorescent molecules.
wildDISCO enables researchers to tag many different types of cells across the roughly 2-centimeter-thick bodies of lab mice. It overcomes a key challenge inherent to previous tissue-clearing techniques such as CUBIC that depend on genetically modified rodents, which are expensive and time-consuming to create and only produce fluorescent proteins in a limited set of cells of interest, such as neurons.
Nerve map: Neuronal projections can be seen at varying depths across the 2-centimeter-thick body of a mouse.
The new method builds on another one Ertürk and his colleagues created called vDISCO, which uses “nanobodies” — antibodies that are one-tenth the usual size — to label targets. vDISCO has helped generate maps of mouse neuron connections stretching all the way from the spinal cord to the toes, but only a handful of nanobodies are available, limiting the approach’s usefulness.
By contrast, wildDISCO uses conventional antibodies, enabling researchers to draw upon countless molecules developed over decades for labeling cells. It relies on a type of cyclodextrin — a ring-shaped sugar molecule — that can remove cholesterol from cell membranes to make them more permeable, enabling conventional antibodies to pervade the entire mouse body.
“Antibody labeling of proteins with a specific function is a mainstay of modern anatomy, yet deep penetration of antibodies into tissue has been difficult, typically forcing scientists into examining only thin slices of tissue,” says David Kleinfeld, professor of physics and neurobiology at the University of California, San Diego, who was not involved in this study. “Ali Ertürk and colleagues have come up with a clever scheme that allows many common antibodies to permeate tissue.”
Thin tubes: Lymphatic vessels course through the mouse body, including some parts of the brain.
Using wildDISCO, the researchers easily imaged nerves coursing through diverse organs, such as the heart and spleen. They also visualized nerve connections between different organs and systems throughout the mouse body, including frequent contact between immune cells and nerve cells on the intestinal wall, and neurons interacting with lymph nodes.
wildDISCO has already yielded unexpected discoveries. For instance, brain tissue was thought to be devoid of lymph vessels, but Ertürk and his colleagues spotted some small ones that descend from the meninges into the brain, and some that connect the cortex to the olfactory bulb.
In addition, germ-free mice — which lack commensal microbes and show increased anxiety-like behavior and impaired social behavior — have a less dense nerve lattice network in the intestinal wall than do their wildtype counterparts, Ertürk and his colleagues found. These findings support previous research suggesting that the microbiome can influence neurons’ development and penetration across the body.
Internal view: The new tissue-clearing technique gives a glimpse of the lymphatic network in the gut of a mouse.
“The technique looks very promising and may well become a powerful new standard procedure, especially combined with 3D microscopy techniques,” says Beth Friedman, a project scientist in computer science and engineering at the University of California, San Diego, who did not work on this study.
So far, Ertürk and his colleagues have validated more than 30 antibodies for use with wildDISCO, and they intend to validate more. They also plan to use artificial-intelligence algorithms to help analyze the vast amounts of data wildDISCO can generate, and develop platforms to share all these data, Ertürk says.
“A major future challenge would be extending the applicability of wildDISCO to human tissues,” he says.
An atlas of high-resolution images of the mouse nervous, lymphatic and vascular systems, and a tutorial video to help examine them, is freely accessible online.
This content was originally published here.